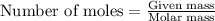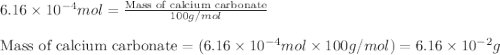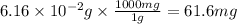Chemistry, 23.07.2019 10:00 coralstoner6793
Acid precipitation dripping on limestone produces carbon dioxide by the following reaction: caco3(s) + 2h+(aq) > ca(2+)(aq) + co2(g) + h2o (l) 15.5ml of co2 was produced at 25*c and 738.0 mmhg how man moles of co2 were produced? how many milligrams of caco3 were consumed?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:40
Which of the following might a chemist choose to study? a. glacier movement in alaska b. better ways to recycle plastics c. the effects of hurricanes on turtle populations d. the vibrations in bridges caused by big trucks
Answers: 2

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1
You know the right answer?
Acid precipitation dripping on limestone produces carbon dioxide by the following reaction: caco3(s...
Questions

Health, 04.02.2021 21:00


History, 04.02.2021 21:00


Chemistry, 04.02.2021 21:00

Advanced Placement (AP), 04.02.2021 21:00

Health, 04.02.2021 21:00

Mathematics, 04.02.2021 21:00


Mathematics, 04.02.2021 21:00




English, 04.02.2021 21:00

Mathematics, 04.02.2021 21:00


History, 04.02.2021 21:00

English, 04.02.2021 21:00


Mathematics, 04.02.2021 21:00

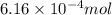 and the mass of calcium carbonate is 61.6 mg
and the mass of calcium carbonate is 61.6 mg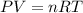
![25^oC=[25+273]K=298K](/tpl/images/0123/0054/df1f6.png)
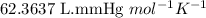

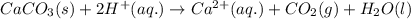
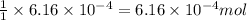 of calcium carbonate
of calcium carbonate