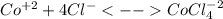In an aqueous chloride solution cobalt(ii) exists in equilibrium with the complex ion cocl42-. co2+(aq) is pink and cocl42-(aq) is blue. at low temperature the pink color predominates. at high temperature the blue color is strong. if we represent the equilibrium as: +(aq) + 4cl-(aq) cocl42-(aq) we can conclude that: 1. this reaction is: a. exothermic b. endothermic c. neutral d. more information is needed to answer this question.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 23.06.2019 04:00
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2

Chemistry, 23.06.2019 07:30
Which of the following statements best explains why chemistry is testable a) it can measure data by experiments b) it cannot add new evidence c) it cannot be verified d) it is biased
Answers: 1

Chemistry, 23.06.2019 16:00
Which is the best metal to use in an alloy to increase its electrical conductivity?
Answers: 2
You know the right answer?
In an aqueous chloride solution cobalt(ii) exists in equilibrium with the complex ion cocl42-. co2+(...
Questions



History, 04.11.2019 19:31




Mathematics, 04.11.2019 19:31


Social Studies, 04.11.2019 19:31

Mathematics, 04.11.2019 19:31

Biology, 04.11.2019 19:31



Mathematics, 04.11.2019 19:31




Physics, 04.11.2019 19:31

Social Studies, 04.11.2019 19:31