
Chemistry, 23.07.2019 16:30 Lydiac9243
How many liters of water must be added to 5.40 g of sodium nitrate to create a solution that has a concentration of 3.81 g/l? use . 0.706 l 1.42 l 1.59 l 20.6 l?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 23.06.2019 04:20
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1

Chemistry, 23.06.2019 06:20
Type the correct answer in each box.balance the chemical equation.__ n203 ➡️ __ n2 +__ o2
Answers: 1

Chemistry, 23.06.2019 07:30
Assignment directions: pick one of the following chemists and perform a bit of research on him/her. answer the following questions. alice hamilton rosalind franklin marie curie gertrude b. elion ada yonath henry cavendish robert boyle antoine lavoisier mario j. molina svante arrhenius
Answers: 1
You know the right answer?
How many liters of water must be added to 5.40 g of sodium nitrate to create a solution that has a c...
Questions


Mathematics, 12.03.2021 01:30

Mathematics, 12.03.2021 01:30


Mathematics, 12.03.2021 01:30

Mathematics, 12.03.2021 01:30

Mathematics, 12.03.2021 01:30

Arts, 12.03.2021 01:30

Mathematics, 12.03.2021 01:30

History, 12.03.2021 01:30



Mathematics, 12.03.2021 01:30


History, 12.03.2021 01:30

Mathematics, 12.03.2021 01:30

Mathematics, 12.03.2021 01:30



Mathematics, 12.03.2021 01:30

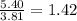 liter of water
liter of water

