
Chemistry, 23.07.2019 19:30 Jocelyn0925
Which of the following statements is true of combustion reactions? a. hydrogen gas (h2) must be one of the reactants b. water (h2o) is always a product c. energy is released by the reaction d. carbon monoxide (co2) is always produced

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1
You know the right answer?
Which of the following statements is true of combustion reactions? a. hydrogen gas (h2) must be one...
Questions



Chemistry, 09.03.2021 01:30





Mathematics, 09.03.2021 01:30


Mathematics, 09.03.2021 01:30

Mathematics, 09.03.2021 01:30





History, 09.03.2021 01:30


Mathematics, 09.03.2021 01:30

Biology, 09.03.2021 01:30

Biology, 09.03.2021 01:30

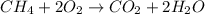
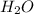 ) is always a product, is true of combustion reactions.
) is always a product, is true of combustion reactions.

