
Chemistry, 23.07.2019 20:00 lovelarissa
Use the following table to answer the question. strong acids weak acids weak bases strong bases hbr ch3cooh nh3 naoh hno3 hf nh4oh koh h2so4 hcn ca(oh)2 which of these is a neutral salt? na2so4 nacn kf (nh4)2so4

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Motivation cannot be developed with practice; a person either possesses it or they do not.
Answers: 1

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3
You know the right answer?
Use the following table to answer the question. strong acids weak acids weak bases strong bases hbr...
Questions







Mathematics, 06.12.2020 23:50




Medicine, 06.12.2020 23:50


Mathematics, 06.12.2020 23:50

English, 06.12.2020 23:50



Business, 06.12.2020 23:50

Chemistry, 06.12.2020 23:50


Biology, 06.12.2020 23:50

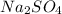
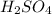 and NaOH which is a strong acid and strong base. Thus, this salt is considered as a neutral salt.
and NaOH which is a strong acid and strong base. Thus, this salt is considered as a neutral salt.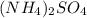
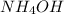 which is a strong acid and weak base. Thus, this salt is considered as an acidic salt.
which is a strong acid and weak base. Thus, this salt is considered as an acidic salt.

