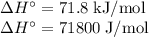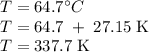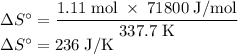Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1
You know the right answer?
The normal boiling point of methanol is 64.7 °c and the molar enthalpy of vaporization if 71.8 kj/mo...
Questions

Mathematics, 29.12.2020 01:30

Mathematics, 29.12.2020 01:30

Mathematics, 29.12.2020 01:30

History, 29.12.2020 01:30

Mathematics, 29.12.2020 01:30

World Languages, 29.12.2020 01:30

Health, 29.12.2020 01:30

Social Studies, 29.12.2020 01:30

Physics, 29.12.2020 01:30




Arts, 29.12.2020 01:30


Mathematics, 29.12.2020 01:30

English, 29.12.2020 01:30

Health, 29.12.2020 01:30

Mathematics, 29.12.2020 01:30


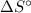 ) has been given as:
) has been given as: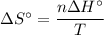
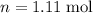
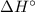 ),
),