Chemistry, 23.07.2019 21:00 janelisse199820
The combustion of gasoline produces carbon dioxide and water. assume gasoline to be pure octane (c8h18) and calculate the mass (in kg) of carbon dioxide that is added to the atmosphere per 1.3 kg of octane burned. (hint: begin by writing a balanced equation for the combustion reaction.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3


Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 23:00
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3
You know the right answer?
The combustion of gasoline produces carbon dioxide and water. assume gasoline to be pure octane (c8h...
Questions



Health, 07.03.2021 20:40

Mathematics, 07.03.2021 20:40

Social Studies, 07.03.2021 20:40





History, 07.03.2021 20:40

Mathematics, 07.03.2021 20:40

Mathematics, 07.03.2021 20:40

Mathematics, 07.03.2021 20:40



Mathematics, 07.03.2021 20:40

Mathematics, 07.03.2021 20:40



Mathematics, 07.03.2021 20:40

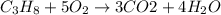
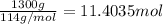
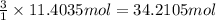 of carbon dioxide
of carbon dioxide