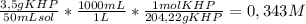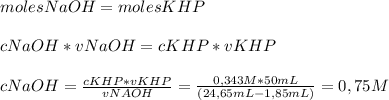To determine the molarity of naoh solution, student took 3.5 g of khp (khp – potassium hydrogen phthalate; molar mass = 204.22 g/mol) and dissolved in 50 ml of water and titrated with the given unknown molarity naoh solution loaded in burette. his burette volume read 1.85 ml at the start of the experiment and 24.65 ml when the phenolphthalein indicator turned pink. using above experimental details, determine the molarity of naoh solution? (enter your answer in two decimal palaces).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2
You know the right answer?
To determine the molarity of naoh solution, student took 3.5 g of khp (khp – potassium hydrogen phth...
Questions

Social Studies, 26.11.2019 06:31






Computers and Technology, 26.11.2019 06:31



Physics, 26.11.2019 06:31