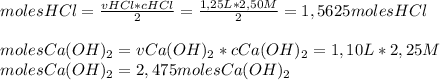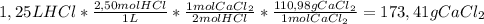Chemistry, 24.07.2019 08:00 freshysans4
How many grams of calcium chloride would form if 1.25 liters of a 2.50 molar hydrochloric acid (hcl) solution reacts with 1.10 liters of a 2.25 molar calcium hydroxide ca(oh)2 solution? (2 points) 2hcl (aq) + ca(oh)2 (aq) yields cacl2 (aq) + h2o (l)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 02:20
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2
You know the right answer?
How many grams of calcium chloride would form if 1.25 liters of a 2.50 molar hydrochloric acid (hcl)...
Questions



English, 03.02.2020 08:43



Chemistry, 03.02.2020 08:43


Mathematics, 03.02.2020 08:43


English, 03.02.2020 08:43

History, 03.02.2020 08:43



Mathematics, 03.02.2020 08:43

Computers and Technology, 03.02.2020 08:43


Mathematics, 03.02.2020 08:43

Mathematics, 03.02.2020 08:43