
Chemistry, 24.07.2019 11:30 AshlynPlayz45
In 1909 fritz haber discovered the workable conditions under which nitrogen, n2(g), and hydrogen, h2(g), would combine using to produce ammonia. the conditions included medium temperature (~500oc), very high pressure (~351kpa), and an iron catalyst. the reaction is represented by the equation: n2(g) + 3h2(g) → 2nh3(g) how many grams of nitrogen are needed to produce 100 grams of ammonia gas?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 23.06.2019 01:00
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2

Chemistry, 23.06.2019 06:00
Amanda pushes a box across the room with a force of 30 n. it accelerates at 5 m/s/s. what is the mass of the box? * 6 kg 1.16 kg 30 kg 5kg
Answers: 2
You know the right answer?
In 1909 fritz haber discovered the workable conditions under which nitrogen, n2(g), and hydrogen, h2...
Questions

History, 30.06.2019 12:30

Health, 30.06.2019 12:30

Spanish, 30.06.2019 12:30



Mathematics, 30.06.2019 12:30



Mathematics, 30.06.2019 12:30

History, 30.06.2019 12:30

Biology, 30.06.2019 12:30


Mathematics, 30.06.2019 12:30


Social Studies, 30.06.2019 12:30


Business, 30.06.2019 12:30

Social Studies, 30.06.2019 12:30

Chemistry, 30.06.2019 12:30

Mathematics, 30.06.2019 12:30

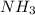 = 100 g
= 100 g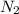 = 28 g/mole
= 28 g/mole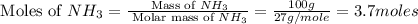
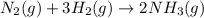
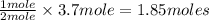 of
of 

