
Chemistry, 24.07.2019 13:00 ArelysMarie
The half-cell is a chamber in the voltaic cell where one half-cell is the site of the oxidation reaction and the other half-cell is the site of the reduction reaction. type the half-cell reaction that takes place at the anode for the chromium-silver voltaic cell. indicate the physical states of atoms and ions using the abbreviation (s), (l), or (g) for solid, liquid, or gas, respectively. use (aq) for an aqueous solution. do not include phases for electrons.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Asample of silver (with work function ? = 4.52 ev) is exposed to an ultraviolet light source (? = 200 nm), which results in the ejection of photoelectrons. what changes will be observed if: silver is replaced with copper (? = 5.10 ev) more photoelectrons ejected no photoelectrons are emitted fewer photoelectrons ejected more energetic photoelectrons (on average) less energetic photoelectrons (on average)
Answers: 3

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2
You know the right answer?
The half-cell is a chamber in the voltaic cell where one half-cell is the site of the oxidation reac...
Questions

Mathematics, 03.02.2021 19:50



Social Studies, 03.02.2021 19:50

Biology, 03.02.2021 19:50





History, 03.02.2021 19:50

Mathematics, 03.02.2021 19:50

History, 03.02.2021 19:50

Mathematics, 03.02.2021 19:50


Mathematics, 03.02.2021 19:50



Mathematics, 03.02.2021 19:50

Mathematics, 03.02.2021 19:50

Mathematics, 03.02.2021 19:50

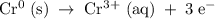 .
.

