
Chemistry, 24.07.2019 18:00 potato3999
The equation below shows the decomposition of lead nitrate. how many grams of oxygen are produced when 11.5 g no2 is formed? 2pb(no3) 2(s) --> 2pbo(s) + 4no2 (g) + o2(g

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2


Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
You know the right answer?
The equation below shows the decomposition of lead nitrate. how many grams of oxygen are produced wh...
Questions


Mathematics, 26.10.2020 23:20


Physics, 26.10.2020 23:20

History, 26.10.2020 23:20

Mathematics, 26.10.2020 23:20


English, 26.10.2020 23:20





Chemistry, 26.10.2020 23:20

Chemistry, 26.10.2020 23:20

Mathematics, 26.10.2020 23:20


Biology, 26.10.2020 23:20

Mathematics, 26.10.2020 23:20

Mathematics, 26.10.2020 23:20

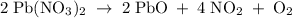
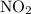 : Oxygen has been 2 : 1.
: Oxygen has been 2 : 1.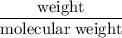
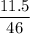
 moles of Oxygen
moles of Oxygen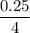
 0.06
0.06

