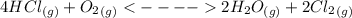1. what would happen to the position of equilibrium when the following changes are made to the reaction below? 2hg3o (g) ↔ 6hg (g) + o2 (g) δh- (a) hg3o is added to the system (b) the volume of the system decreases (c) temperature is increased 2. balance the exothermic reaction no2 ↔ n2o4 kj – 58.0kj predict the effect of each of the following changes on this system at equilibrium (a) added n2o4 (b) increase the volume (c) add n2 (d) remove no2 (e) decrease the temperature 3. + 333 kj 2no2 ↔ n2 + o2 (a) no2 is added to the system (b) n2 is added to the system (c) o2 is removed from the system (d) the temperature of the container increased (e) the volume of the container increase (f) n2 is added and no2 is removed 4. 179 kj caco3 ↔ cao + co2 (a) co2 is added (b) the volume of the container decreased (c) cao is removed from the system (d) the temperature of the container decreased (e) the volume of the container is increased. (f) caco3 is added to the system 5. h2 + cl2 ↔ 2hcl (a) h2 is removed from the system (b) hcl is removed from the system (c) the volume of the container is removed (d) the temperature of the container is increased (e) the concentration of cl2 is decreased (f) the volume of the container decreased 6. list all the “stressors” that could be applied to this equilibrium reaction, which would cause an increase in the concentration of water vapor. 4hcl (g) + o2 (g) ↔ 2h2o (g) + 2cl2 (g)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:00
How are ionic bonds formed and what is the attractive force within an ionic bond
Answers: 1

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 23.06.2019 09:20
Asolution of naoh has a concentration of 25.00% by mass. what mass of naoh is present in 0.250 g of this solution? use the periodic table in the toolbar if needed. 0.0625 g what mass of naoh must be added to the solution to increase the concentration to 30.00% by mass? g
Answers: 2
You know the right answer?
1. what would happen to the position of equilibrium when the following changes are made to the react...
Questions












Health, 09.03.2020 21:45



Mathematics, 09.03.2020 21:46


Mathematics, 09.03.2020 21:46

English, 09.03.2020 21:47



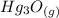 is 272.1 KJ/mol & std heat of formation of Hg is 61.38 KJ/mol,
is 272.1 KJ/mol & std heat of formation of Hg is 61.38 KJ/mol, 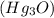
 .
. 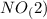 will give 1 dinitrogen tetraoxide.
will give 1 dinitrogen tetraoxide. 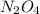 is added to the rxn the rxn eqm will be shifted to <--.
is added to the rxn the rxn eqm will be shifted to <--. 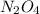 will Incr. the prod & so it will shift the eqm to the backward rxn to produce more reactants.
will Incr. the prod & so it will shift the eqm to the backward rxn to produce more reactants. 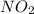 the eqm will shift to <--.
the eqm will shift to <--.  balanced rxn.
balanced rxn. 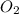 is removed from the rxn then eqm will shift on -->.
is removed from the rxn then eqm will shift on -->. 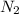 is added &
is added &  balanced equation. Endo rxn as ΔH is +ve = 179 KJ.
balanced equation. Endo rxn as ΔH is +ve = 179 KJ. 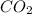 is added to the rxn then the eqm shifts to <--.
is added to the rxn then the eqm shifts to <--. 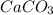 is added will shift to -->.
is added will shift to -->.  It is the balanced equation & is exo rxn.
It is the balanced equation & is exo rxn.