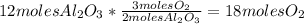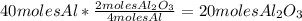Chemistry, 25.07.2019 01:00 ethanhose05
Balance this reaction: al(s) + o2(g) → al2o3(s) how many moles of oxygen will be needed to react with aluminum metal to produce 12.0 moles of aluminum oxide? 6. using the reaction listed in question 5 (above), how many moles of aluminum oxide will be produced by 40.0 moles of aluminum reacting completely with a boat load (that is a lot) of oxygen?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1
You know the right answer?
Balance this reaction: al(s) + o2(g) → al2o3(s) how many moles of oxygen will be needed to react w...
Questions

Mathematics, 16.09.2020 14:01

Social Studies, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

English, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

History, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01