
Chemistry, 25.07.2019 03:00 ewymer3901
Some antacid tablets contain aluminum hydroxide. the aluminum hydroxide reacts with stomach acid according to the equation: ai(oh)3 +3hciaici, +3h2o. determine the moles of acid neutralized if a tablet contains 0.200 mol of al(oh)3

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Consider the point on the plot where 10.0 g of naoh have been added. what amount of naoh, in moles, has been added? 0.308 mol fecl3 initially present
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3

Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1
You know the right answer?
Some antacid tablets contain aluminum hydroxide. the aluminum hydroxide reacts with stomach acid acc...
Questions

English, 14.10.2019 05:20







Social Studies, 14.10.2019 05:20

Social Studies, 14.10.2019 05:20


Biology, 14.10.2019 05:20

Health, 14.10.2019 05:20

Mathematics, 14.10.2019 05:20

English, 14.10.2019 05:20

Biology, 14.10.2019 05:20



Mathematics, 14.10.2019 05:20


Biology, 14.10.2019 05:20

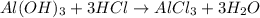
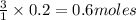 of hydrochloric acid.
of hydrochloric acid.

