Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 2

Chemistry, 23.06.2019 17:30
With carbon dioxide what phase change take place when the temperature decreases from -40c to -80c at 2 atm
Answers: 2

Chemistry, 23.06.2019 19:10
Is the involuntary movement of the muscles that move food through the digestive system.
Answers: 1
You know the right answer?
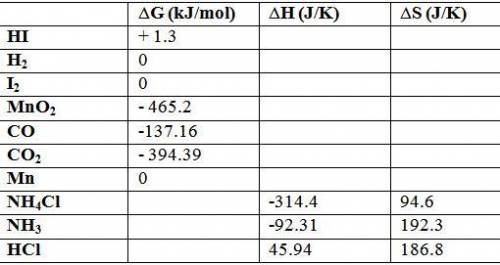
Calculate δg o for each reaction using δg of values: (a) h2(g) + i2(s) → 2hi(g) kj (b) mno2(s) + 2co...
Questions




English, 24.09.2019 00:40



Biology, 24.09.2019 00:40



Business, 24.09.2019 00:40

Biology, 24.09.2019 00:40



Mathematics, 24.09.2019 00:40

Mathematics, 24.09.2019 00:40


History, 24.09.2019 00:40