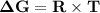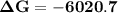Chemistry, 25.07.2019 07:00 zetrenne73
Consider the reaction 2no2(g) → n2o4(g) and δg∘(no2(g)) = 51.84 kj/mol, δg∘(n2o4(g)) = 98.28 kj/mol. calculate δg at 298 k if the partial pressures of no2 and n2o4 are 0.38atm and 1.64atm , respectively.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

You know the right answer?
Consider the reaction 2no2(g) → n2o4(g) and δg∘(no2(g)) = 51.84 kj/mol, δg∘(n2o4(g)) = 98.28 kj/mol....
Questions

















Mathematics, 10.11.2019 01:31

Mathematics, 10.11.2019 01:31