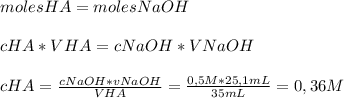Chemistry, 25.07.2019 10:00 tashatyron24pejls0
Determine the concentration of 35.0ml of an acid solution that requires 25.1ml of a 0.50m naoh to neutralize it

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is important to study for nios grade 12 chemistry? i have only one month left.
Answers: 2


Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 23.06.2019 02:30
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2
You know the right answer?
Determine the concentration of 35.0ml of an acid solution that requires 25.1ml of a 0.50m naoh to ne...
Questions




Social Studies, 02.11.2019 23:31

Mathematics, 02.11.2019 23:31






Geography, 02.11.2019 23:31


Biology, 02.11.2019 23:31


Mathematics, 02.11.2019 23:31





Geography, 02.11.2019 23:31