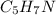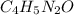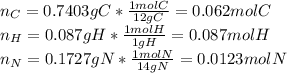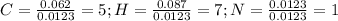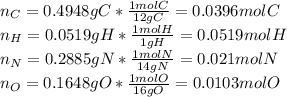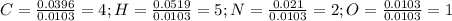Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is the nature of the ca-cl bond in a molecule of calcium chloride (cacl2) if the electronegativity value of calcium is 1.0 and that of chlorine is 3.16?
Answers: 1

Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 23.06.2019 06:00
Amanda pushes a box across the room with a force of 30 n. it accelerates at 5 m/s/s. what is the mass of the box? * 6 kg 1.16 kg 30 kg 5kg
Answers: 2
You know the right answer?
Calculate the empirical formula for each stimulant based on its elemental mass percent composition....
Questions

Computers and Technology, 12.07.2019 21:00


History, 12.07.2019 21:00

Mathematics, 12.07.2019 21:00



Social Studies, 12.07.2019 21:00


Biology, 12.07.2019 21:00





English, 12.07.2019 21:00

Mathematics, 12.07.2019 21:00


Advanced Placement (AP), 12.07.2019 21:00



Social Studies, 12.07.2019 21:00