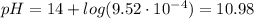Chemistry, 25.07.2019 16:00 Tyrant4life
For the titration of 50.0 ml of 0.020 m aqueous salicylic acid with 0.020 m koh (aq), calculate the ph after the addition of 55.0 ml of the base. for salycylic acid, pka = 2.97.'

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2
You know the right answer?
For the titration of 50.0 ml of 0.020 m aqueous salicylic acid with 0.020 m koh (aq), calculate the...
Questions


Computers and Technology, 03.03.2020 01:55


Mathematics, 03.03.2020 01:55





Mathematics, 03.03.2020 01:55

Biology, 03.03.2020 01:55



Mathematics, 03.03.2020 01:55



History, 03.03.2020 01:56




Mathematics, 03.03.2020 01:56

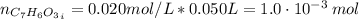
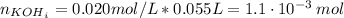
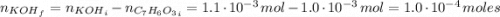
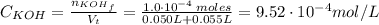
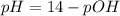
![pH = 14 - (-log[OH^{-}])](/tpl/images/0131/6319/7f1c5.png)