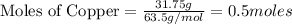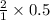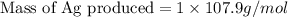Chemistry, 25.07.2019 17:30 michaellagann2020
Copper metal (cu) reacts with silver nitrate (agno3) in aqueous solution to form ag and cu(no3)2. an excess of agno3 is present. the balanced chemical equation is shown below. cu + 2agno3 mc021-1.jpg cu(no3)2 + 2ag the molar mass of cu is 63.5 g/mol. the molar mass of ag is 107.9 g/mol. what mass, in grams, of ag is produced from reaction of 31.75 g of cu? 26.95 107.9 215.91 431.82

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2



You know the right answer?
Copper metal (cu) reacts with silver nitrate (agno3) in aqueous solution to form ag and cu(no3)2. an...
Questions

History, 16.09.2019 05:30

English, 16.09.2019 05:30


Geography, 16.09.2019 05:30

Biology, 16.09.2019 05:30


Physics, 16.09.2019 05:30


Social Studies, 16.09.2019 05:30

History, 16.09.2019 05:30


Mathematics, 16.09.2019 05:30

English, 16.09.2019 05:30


History, 16.09.2019 05:30


Physics, 16.09.2019 05:30

Mathematics, 16.09.2019 05:30

English, 16.09.2019 05:30

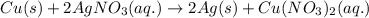
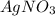 is present in excess, therefore Copper metal is considered as the limiting reagent because it limits the formation of product.
is present in excess, therefore Copper metal is considered as the limiting reagent because it limits the formation of product.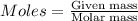 ....(1)
....(1)