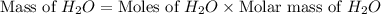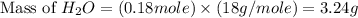Gaseous methane ch4 will react with gaseous oxygen o2 to produce gaseous carbon dioxide co2 and gaseous water h2o . suppose 1.44 g of methane is mixed with 9.5 g of oxygen. calculate the maximum mass of water that could be produced by the chemical reaction. round your answer to 3 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Achemist requires 6.00 liters of 0.320 m h2so4 solution. how many grams of h2so4 should the chemist dissolve in water? 121 grams 159 grams 176 grams 188 grams
Answers: 2

Chemistry, 21.06.2019 19:00
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 23.06.2019 07:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1
You know the right answer?
Gaseous methane ch4 will react with gaseous oxygen o2 to produce gaseous carbon dioxide co2 and gase...
Questions



Mathematics, 06.12.2021 20:40

Biology, 06.12.2021 20:40


English, 06.12.2021 20:40


Mathematics, 06.12.2021 20:40

Mathematics, 06.12.2021 20:40

Mathematics, 06.12.2021 20:40

Mathematics, 06.12.2021 20:40




English, 06.12.2021 20:40


English, 06.12.2021 20:40



Mathematics, 06.12.2021 20:40

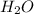 produced will be, 3.24 grams
produced will be, 3.24 grams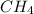 = 1.44 g
= 1.44 g
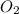 = 9.5 g
= 9.5 g
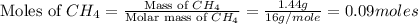
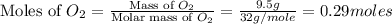
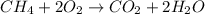
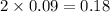 moles of
moles of