

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 06:30
Acompound has the molecular formula c3h8. which class of organic compounds does it belong to?
Answers: 1


Chemistry, 23.06.2019 13:30
The activation energy for a(n) is quite large and usually takes extra energy from the environment, it is normally not a natural spontaneous process. combustion reaction endothermic reaction exothermic reaction catalyzed reaction
Answers: 3

Chemistry, 23.06.2019 17:00
Identify the missing coefficient in the balanced equation and classify the type of reaction. mg(oh)2 + h2so4 ⟶ mgso4 + 1; combustion 1; neutralization 2; combustion 2; neutralization
Answers: 1
You know the right answer?
Asample of radium-226 decays to form radon-222. a solution of agno3 and nacl reacts to form agcl and...
Questions





Social Studies, 25.02.2022 14:40






Social Studies, 25.02.2022 14:40







Spanish, 25.02.2022 14:50


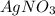 and NaCl will increase the reaction rate as the reaction follows first order kinetics with respect to
and NaCl will increase the reaction rate as the reaction follows first order kinetics with respect to 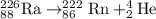
![rate= k[Ra]^1](/tpl/images/0132/6328/f5c02.png)
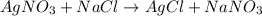
![rate= k[AgNO_3]^1[NaCl]^1](/tpl/images/0132/6328/ac277.png)


