
Chemistry, 26.07.2019 01:00 keelyrosewillia
How many moles of n2o5 are needed to produce 7.90 g of no2? 2n2o5 = 4no2 + o2

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1


Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
You know the right answer?
How many moles of n2o5 are needed to produce 7.90 g of no2? 2n2o5 = 4no2 + o2...
Questions




Chemistry, 21.05.2020 20:07




English, 21.05.2020 20:07


English, 21.05.2020 20:07


Biology, 21.05.2020 20:07

Mathematics, 21.05.2020 20:07

Mathematics, 21.05.2020 20:07



History, 21.05.2020 20:07



History, 21.05.2020 20:07

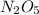 is needed to produce 7.9 grams of
is needed to produce 7.9 grams of 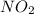
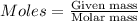
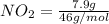 = 0.1717 moles
= 0.1717 moles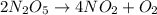
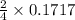 = 0.08585 moles of
= 0.08585 moles of 

