
Chemistry, 26.09.2019 01:00 fickllyd000
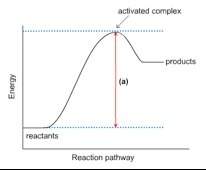
1. this energy diagram is for the thermal decomposition of solid mercury (ii) oxide (also known as mercuric oxide) into liquid mercury and oxygen gas.
• write a balanced equation for the reaction.
• explain what feature is shown by the arrow labeled (a).
• using chemical symbols and dashed lines (this can be done with type), draw what the activated complex or transition state might look like.
• is this reaction exothermic or endothermic? explain.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:20
Determine which intermolecular forces are the dominant (strongest) forces for a pure sample of each of the following molecules by placing the molecules into the correct bins. drag the appropriate molecular formula to their respective bins.
Answers: 3

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
1. this energy diagram is for the thermal decomposition of solid mercury (ii) oxide (also known as m...
Questions


Computers and Technology, 26.02.2020 23:21

Computers and Technology, 26.02.2020 23:21

History, 26.02.2020 23:22

Geography, 26.02.2020 23:22








Mathematics, 26.02.2020 23:22




Mathematics, 26.02.2020 23:22

English, 26.02.2020 23:22

History, 26.02.2020 23:22




