Chemistry, 19.09.2019 07:50 lacourboud20005
When 8.00 × 1022 molecules of ammonia react with 7.00 × 1022 molecules of oxygen according to the chemical equation shown below, how many grams of nitrogen gas are produced?
4 nh3(g) + 3 o2(g) → 2 n2(g) + 6 h2o(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Alarge marble is dropped in a graduated cylinder with 35ml of water in it.the water level increases to 49ml.what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3
You know the right answer?
When 8.00 × 1022 molecules of ammonia react with 7.00 × 1022 molecules of oxygen according to the ch...
Questions



Social Studies, 02.09.2019 17:10




Mathematics, 02.09.2019 17:10

Geography, 02.09.2019 17:10

Mathematics, 02.09.2019 17:10


History, 02.09.2019 17:10



Biology, 02.09.2019 17:10

Computers and Technology, 02.09.2019 17:10


Mathematics, 02.09.2019 17:20

History, 02.09.2019 17:20

Spanish, 02.09.2019 17:20

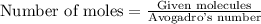 ....(1)
....(1)
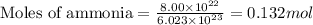
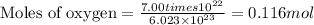
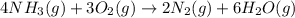
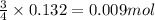 of oxygen
of oxygen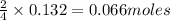 of nitrogen
of nitrogen