Chemistry, 26.07.2019 15:00 victorialeona81
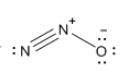
Which resonance form is likely to contribute most to the correct structure of n2o? which resonance form is likely to contribute most to the correct structure of ? structure for nno showing three lone-pairs of electrons on the terminal nitrogen atom, a single bond between the two nitrogen atoms, a triple bond between nitrogen and oxygen, and one lone-pair of electrons on the terminal oxygen atom. structure for nno showing two lone-pairs of electrons on the terminal nitrogen atom, a double bond between the two nitrogen atoms, a double bond between nitrogen and oxygen, and two lone-pairs of electrons on the terminal oxygen atom. structure for nno showing one lone-pair of electrons on the terminal nitrogen atom, a triple bond between the two nitrogen atoms, a single bond between nitrogen and oxygen, and three lone-pairs of electrons on the terminal oxygen atom?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1


Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2
You know the right answer?
Which resonance form is likely to contribute most to the correct structure of n2o? which resonance...
Questions


English, 27.09.2021 16:10

Physics, 27.09.2021 16:10

History, 27.09.2021 16:10



Mathematics, 27.09.2021 16:10

English, 27.09.2021 16:10

Geography, 27.09.2021 16:10


Mathematics, 27.09.2021 16:10

Mathematics, 27.09.2021 16:10


English, 27.09.2021 16:10

English, 27.09.2021 16:10

Computers and Technology, 27.09.2021 16:10



Mathematics, 27.09.2021 16:10

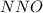 is:
is: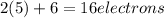
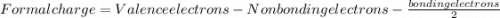
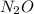 is:
is: