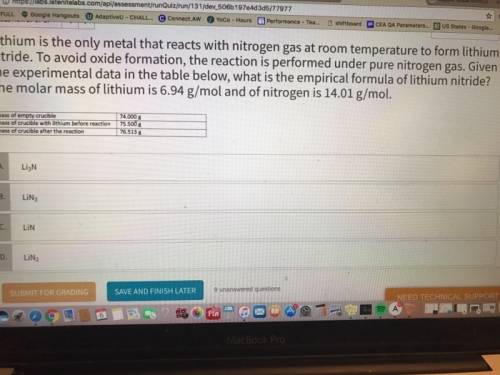
Lithium is the only metal that reacts with nitrogen gas at room temperature to form lithium nitride. to avoid oxide formation, the reaction is performed under pure nitrogen gas. given the experimental data in the table below, what is the empirical formula of lithium nitride? the molar mass of lithium is 6.94 g/mol and of nitrogen is 14.01 g/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3

Chemistry, 23.06.2019 04:40
[01.07]what is the answer to the problem: 101 g + 25.01 g + 5.05 g? 131.06 g 131.1 g 131 g 130 g
Answers: 1

Chemistry, 23.06.2019 06:00
Give one example of a pure (exact) number and of an estimated (measured) number.
Answers: 2
You know the right answer?
Lithium is the only metal that reacts with nitrogen gas at room temperature to form lithium nitride....
Questions

Mathematics, 06.11.2020 19:10


Mathematics, 06.11.2020 19:10

History, 06.11.2020 19:10

Business, 06.11.2020 19:10

Mathematics, 06.11.2020 19:10

Mathematics, 06.11.2020 19:10

Social Studies, 06.11.2020 19:10

Mathematics, 06.11.2020 19:10

Mathematics, 06.11.2020 19:10




Mathematics, 06.11.2020 19:10

History, 06.11.2020 19:10


Mathematics, 06.11.2020 19:10

English, 06.11.2020 19:10

Health, 06.11.2020 19:10

Geography, 06.11.2020 19:10