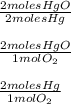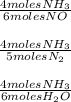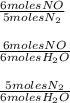Chemistry, 26.07.2019 18:00 alexacarillo
Reviewing main ideas 1. what is stoichiometry? 2. for each equation, write all possible mole ratios. a. 2hgo(s) → 2hg(l) + o2(g) b. 4nh3(g) + 6no(g) → 5n2(g) + 6h2o(l) 3. how is a mole ratio used in stoichiometry? critical thinking 4. relating ideas what step must be performed before any stoichiometry problem is solved? explain.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2
You know the right answer?
Reviewing main ideas 1. what is stoichiometry? 2. for each equation, write all possible mole ratios...
Questions



History, 19.05.2021 20:00





Mathematics, 19.05.2021 20:00


Biology, 19.05.2021 20:00

Mathematics, 19.05.2021 20:00

Mathematics, 19.05.2021 20:00



Mathematics, 19.05.2021 20:00

Mathematics, 19.05.2021 20:00




Mathematics, 19.05.2021 20:00