
Chemistry, 26.07.2019 20:30 wyattgrubb00
Which equivalence factor set should you use to convert from 4.23 x 1012 atoms of ca to grams of ca? a) (1 mol ca/6.02 x 1023 atoms ca)(40.08 g ca/1 mol ca) b) (1 mol ca/4.23 x 1012 atoms ca)(40.08 g ca/1 mol ca) c) (4.23 x 1012 atoms ca/1 mol ca)(1 mol ca/40.08 g ca) d) 4.23 x 1012 atoms ca/6.02 x 1023 atoms ca)(40.08 g ca)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2


You know the right answer?
Which equivalence factor set should you use to convert from 4.23 x 1012 atoms of ca to grams of ca?...
Questions




Health, 30.07.2019 05:30

Social Studies, 30.07.2019 05:30



Social Studies, 30.07.2019 05:30


History, 30.07.2019 05:30


Physics, 30.07.2019 05:30

Health, 30.07.2019 05:30


Arts, 30.07.2019 05:30


Social Studies, 30.07.2019 05:30



Biology, 30.07.2019 05:30

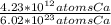 * 40.08 g Ca
* 40.08 g Ca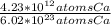 * 40.08 g Ca
* 40.08 g Ca

