
Chemistry, 26.07.2019 20:30 lilsmitty137ovf7yw
Using the heat equation q=mc∆t, what would the formula look like if we were solving for change in temperature? a mcq=∆t b q/mc= ∆t c qm/c=∆t d q m=∆tc give me a reason why and go into detail.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 23.06.2019 01:00
Which statement is true regarding the diagram of circle p? the sum of y and z must be 2x. the sum of y and z must be x. the difference of z and y must be 2x. the difference of z and y must be x
Answers: 1

Chemistry, 23.06.2019 08:00
At 35.0°c and 3.00 atm pressure, a gas has a volume of 1.40 l. what pressure does the gas have at 0.00°c and a volume of 0.950 l? which equation should you use? p2= p1v1t2/t1v2what is the pressure of the gas? 3.92 atm these are the answers
Answers: 1
You know the right answer?
Using the heat equation q=mc∆t, what would the formula look like if we were solving for change in te...
Questions

Chemistry, 19.03.2021 20:40


Mathematics, 19.03.2021 20:40


Mathematics, 19.03.2021 20:40

Health, 19.03.2021 20:40


Mathematics, 19.03.2021 20:40


Mathematics, 19.03.2021 20:40


Mathematics, 19.03.2021 20:40





Biology, 19.03.2021 20:40

Mathematics, 19.03.2021 20:40

Chemistry, 19.03.2021 20:40

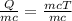 = divide both sides by mc
= divide both sides by mc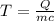 - simplified
- simplified


