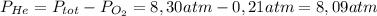He partial pressure of oxygen gas in our atmosphere is 0.21 atm. this is the partial pressure at which human lungs have evolved to be able to breathe this gas. a scuba diver, will thus still have to breath oxygen at this pressure even when diving way down in the water. if a mixture of helium and oxygen (heliox) in his tank is at a pressure of 8.30 atm, what must the partial pressure be of helium to keep the partial pressure of oxygen at 0.21 atm?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Identify which properties could correspond to solids, plasmas, or both. maintain a unique shape. collide infrequently with other particles. have very high velocities. conduct electricity. protons. have a low temperature. has long-range order.
Answers: 1

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2
You know the right answer?
He partial pressure of oxygen gas in our atmosphere is 0.21 atm. this is the partial pressure at whi...
Questions

History, 10.10.2019 05:00


Mathematics, 10.10.2019 05:00

Social Studies, 10.10.2019 05:00


History, 10.10.2019 05:00

Mathematics, 10.10.2019 05:00

Social Studies, 10.10.2019 05:00


Mathematics, 10.10.2019 05:00


Mathematics, 10.10.2019 05:00