
Chemistry, 27.07.2019 01:00 mamieengler
The equation below shows hydrogen reacting with oxygen to produce water. 2h2+o2> 2h2o if 16mol of oxygen were reacted with excess hydrogen gas, how many moles of water would be produced?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1
You know the right answer?
The equation below shows hydrogen reacting with oxygen to produce water. 2h2+o2> 2h2o if 16mol of...
Questions

History, 18.05.2021 17:10

Chemistry, 18.05.2021 17:10

History, 18.05.2021 17:10



History, 18.05.2021 17:10

Chemistry, 18.05.2021 17:10



Mathematics, 18.05.2021 17:10

Mathematics, 18.05.2021 17:10


History, 18.05.2021 17:10

Mathematics, 18.05.2021 17:10


English, 18.05.2021 17:10


Mathematics, 18.05.2021 17:10

Mathematics, 18.05.2021 17:10

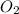 = 16 mole
= 16 mole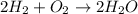
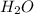
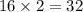 moles of
moles of 

