
Chemistry, 27.07.2019 05:30 timothymoles
The theoretical yield for a reaction is 55.9 g licl. the actual yield is 24.6 g licl. what is the percent yield of the reaction? question options: 227% 44% 25% 56%

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 23.06.2019 01:30
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1

Chemistry, 23.06.2019 04:00
Which method would be best to separate a mixture of sand and gravel
Answers: 1
You know the right answer?
The theoretical yield for a reaction is 55.9 g licl. the actual yield is 24.6 g licl. what is the pe...
Questions




Mathematics, 06.06.2020 01:59

Physics, 06.06.2020 01:59

Engineering, 06.06.2020 01:59




English, 06.06.2020 01:59




Mathematics, 06.06.2020 01:59


Mathematics, 06.06.2020 01:59

Mathematics, 06.06.2020 01:59

Mathematics, 06.06.2020 01:59

Medicine, 06.06.2020 01:59

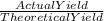 × 100
× 100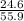 × 100
× 100

