Chemistry, 27.07.2019 06:30 ddddre3909
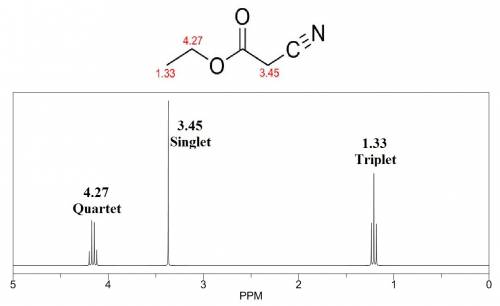
Identify the compound: molecular mass 113; gives a positive hydroxamate test, which indicates the presence of either an acyl chloride or an ester; ir: 2237, 1733, 1200 cm–1; 1h nmr: δ 1.33 (3h, t, j = 7 hz), δ 3.45 (2h, s), δ 4.27 (2h, q, j = 7 hz).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

You know the right answer?
Identify the compound: molecular mass 113; gives a positive hydroxamate test, which indicates the...
Questions




Mathematics, 10.11.2019 03:31


History, 10.11.2019 03:31

Mathematics, 10.11.2019 03:31

Mathematics, 10.11.2019 03:31

Mathematics, 10.11.2019 03:31


Mathematics, 10.11.2019 03:31

Computers and Technology, 10.11.2019 03:31


English, 10.11.2019 03:31



Mathematics, 10.11.2019 03:31



History, 10.11.2019 03:31