Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1



Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1
You know the right answer?
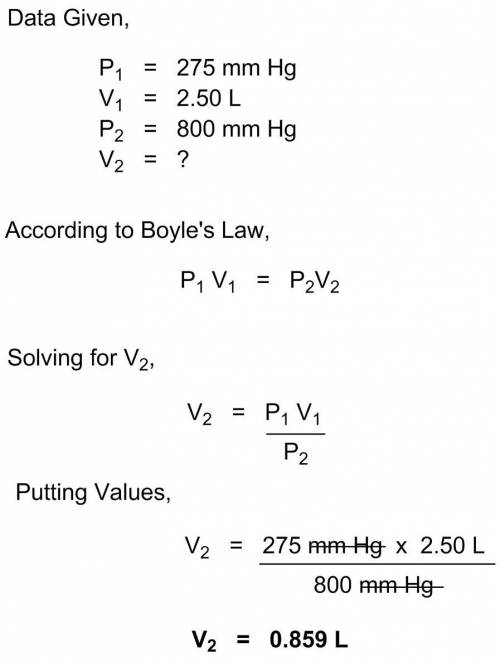
The pressure of a gas is 275 mm hg when it is confined in a 2.50 l container. what would be the volu...
Questions

Mathematics, 25.06.2019 09:30


Chemistry, 25.06.2019 09:30


Mathematics, 25.06.2019 09:30

English, 25.06.2019 09:30

Mathematics, 25.06.2019 09:30

Health, 25.06.2019 09:30

English, 25.06.2019 09:30





Mathematics, 25.06.2019 09:30




Mathematics, 25.06.2019 09:30