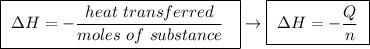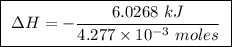Chemistry, 27.07.2019 07:00 alejandra216
Abomb calorimeter has a heat capacity of 2.47 kj/k. when a 0.120-g sample of ethylene (c2h4) was burned in this calorimeter, the temperature increased by 2.44 k. calculate the enthalpy change per mole of ethylene combusted.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

You know the right answer?
Abomb calorimeter has a heat capacity of 2.47 kj/k. when a 0.120-g sample of ethylene (c2h4) was bur...
Questions



Mathematics, 19.04.2021 19:30

Mathematics, 19.04.2021 19:30


English, 19.04.2021 19:30


Biology, 19.04.2021 19:30

Mathematics, 19.04.2021 19:30

Mathematics, 19.04.2021 19:30

Social Studies, 19.04.2021 19:30

Mathematics, 19.04.2021 19:30

History, 19.04.2021 19:30

Biology, 19.04.2021 19:30

Computers and Technology, 19.04.2021 19:30




Chemistry, 19.04.2021 19:30

Mathematics, 19.04.2021 19:30

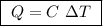
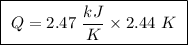
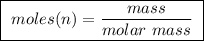
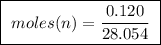
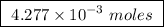 of ethylene.
of ethylene.