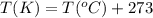Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3
You know the right answer?
At what temperature (in c. does 121 ml of co2 at 27c and 1.05 atm occupy a volume of 293 ml at a pre...
Questions


History, 21.04.2021 14:00


Mathematics, 21.04.2021 14:00


English, 21.04.2021 14:00






English, 21.04.2021 14:00



Computers and Technology, 21.04.2021 14:00

Chemistry, 21.04.2021 14:00


History, 21.04.2021 14:00

Mathematics, 21.04.2021 14:00

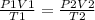

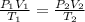
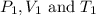 are the initial pressure, volume and temperature of the gas
are the initial pressure, volume and temperature of the gas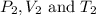 are the final pressure, volume and temperature of the gas
are the final pressure, volume and temperature of the gas![P_1=1.05atm\\V_1=121mL\\T_1=27^oC=[27+273]K=300K\\P_2=1.40atm\\V_2=293mL\\T_2=?K](/tpl/images/0138/4629/aafe7.png)