
Chemistry, 27.07.2019 12:00 thegent1859
Asaturated solution of ag2cro4 has a cro42- concentration of 6.7 x 10-5 m. calculate the molarity of ag+ if the ksp for ag2cro4 is equal to 1.2 x 10-12

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1
You know the right answer?
Asaturated solution of ag2cro4 has a cro42- concentration of 6.7 x 10-5 m. calculate the molarity of...
Questions

Mathematics, 23.08.2019 01:40

History, 23.08.2019 01:40








Business, 23.08.2019 01:40


Physics, 23.08.2019 01:40

History, 23.08.2019 01:40


Mathematics, 23.08.2019 01:40


French, 23.08.2019 01:40

History, 23.08.2019 01:40

German, 23.08.2019 01:40

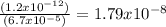
![[Ag^{+}] = \sqrt{1.79 x 10^{-8}} = 1.35 x 10^{-4}](/tpl/images/0138/7009/3c747.png)


