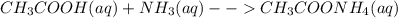Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

You know the right answer?
For which type of titration will the ph be acidic at the equivalence point? a. strong acid vs. stro...
Questions

English, 18.11.2020 18:40


Mathematics, 18.11.2020 18:40

English, 18.11.2020 18:40

English, 18.11.2020 18:40

History, 18.11.2020 18:40






Mathematics, 18.11.2020 18:40

English, 18.11.2020 18:40

Mathematics, 18.11.2020 18:40

Chemistry, 18.11.2020 18:40

Mathematics, 18.11.2020 18:40

Mathematics, 18.11.2020 18:40