
Chemistry, 27.07.2019 23:00 donbright100
Write an equation to show how hco3− can act as a base with hs− acting as an acid.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2


Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 22.06.2019 19:20
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1
You know the right answer?
Write an equation to show how hco3− can act as a base with hs− acting as an acid....
Questions

Computers and Technology, 15.01.2021 05:30

Mathematics, 15.01.2021 05:30



Health, 15.01.2021 05:30

Mathematics, 15.01.2021 05:30

History, 15.01.2021 05:30

Mathematics, 15.01.2021 05:30

English, 15.01.2021 05:30

Arts, 15.01.2021 05:30


French, 15.01.2021 05:30

Mathematics, 15.01.2021 05:30

Physics, 15.01.2021 05:30


History, 15.01.2021 05:30

Mathematics, 15.01.2021 05:30


Mathematics, 15.01.2021 05:30

Biology, 15.01.2021 05:30

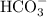 acts as base with
acts as base with 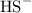 as an acid is
as an acid is 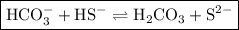
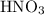 and
and 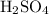 while NaOH and KOH are examples of Arrhenius bases.
while NaOH and KOH are examples of Arrhenius bases.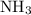 is a Bronsted base.
is a Bronsted base.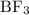 ,
, 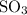 while
while 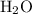 and ROH are the examples of Lewis base.
and ROH are the examples of Lewis base.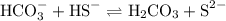
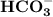 accepts a proton and becomes
accepts a proton and becomes 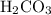 whereas
whereas 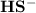 donates a proton and forms
donates a proton and forms 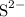 . According to Bronsted-Lowry theory, any species that accepts proton is called base while the one that donates proton acts as an acid.
. According to Bronsted-Lowry theory, any species that accepts proton is called base while the one that donates proton acts as an acid. 

