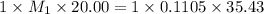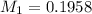You are given a solution of hcooh (formic acid) with an approximate concentration of 0.20 m and you will titrate this with a 0.1105 m naoh. you add 20.00 ml of hcooh to the beaker before titrating, and it requires 35.43 ml of naoh to reach the end point. what is the concentration of the hcooh solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1

Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1
You know the right answer?
You are given a solution of hcooh (formic acid) with an approximate concentration of 0.20 m and you...
Questions

Biology, 07.12.2020 20:40


Mathematics, 07.12.2020 20:40

Mathematics, 07.12.2020 20:40


Social Studies, 07.12.2020 20:40




Mathematics, 07.12.2020 20:40





English, 07.12.2020 20:40

Chemistry, 07.12.2020 20:40

Business, 07.12.2020 20:40

Social Studies, 07.12.2020 20:40

Mathematics, 07.12.2020 20:40

Biology, 07.12.2020 20:40


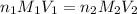
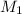 = molarity of
= molarity of 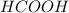 solution = ?
solution = ?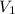 = volume of
= volume of 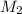 = molarity of
= molarity of 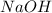 solution = 0.1105 M
solution = 0.1105 M
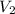 = volume of
= volume of 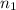 = valency of
= valency of 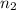 = valency of
= valency of