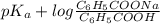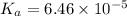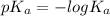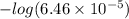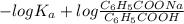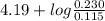Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 01:10
Volume is a measurement of how fast particles of a substance are moving
Answers: 3

Chemistry, 23.06.2019 03:00
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 2

Chemistry, 23.06.2019 06:30
Acompound has the molecular formula c3h8. which class of organic compounds does it belong to?
Answers: 1

Chemistry, 23.06.2019 18:20
Ahydrogen electron is elevated from level 1 to level 2. another electron is elevated from level 2 to level 4. the transition requiring the greatest energy change is?
Answers: 1
You know the right answer?
Calculate the ph of a solution that is 0.115m benzoic acid and 0.230m sodium benzoate, a salt whose...
Questions


Mathematics, 05.03.2021 01:30

Biology, 05.03.2021 01:30


Arts, 05.03.2021 01:30

Computers and Technology, 05.03.2021 01:30

Mathematics, 05.03.2021 01:30


Computers and Technology, 05.03.2021 01:30



Mathematics, 05.03.2021 01:30







Mathematics, 05.03.2021 01:30

Chemistry, 05.03.2021 01:30