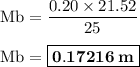Chemistry, 28.07.2019 07:00 carolineepoolee84
A25.00 −ml sample of an unknown hclo4 solution requires titration with 21.52 ml of 0.2000 m naoh to reach the equivalence point. part a what is the concentration of the unknown hclo4 solution? the neutralization reaction is: hclo4(aq)+naoh(aq)→h2o(l)+naclo4(aq )

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2


Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1
You know the right answer?
A25.00 −ml sample of an unknown hclo4 solution requires titration with 21.52 ml of 0.2000 m naoh to...
Questions

Mathematics, 11.06.2021 21:20


Mathematics, 11.06.2021 21:20



Computers and Technology, 11.06.2021 21:20

Social Studies, 11.06.2021 21:20

Business, 11.06.2021 21:20

Mathematics, 11.06.2021 21:20

Spanish, 11.06.2021 21:20






Mathematics, 11.06.2021 21:20

Biology, 11.06.2021 21:20


History, 11.06.2021 21:20