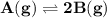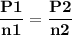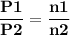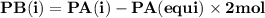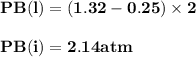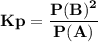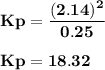Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2
You know the right answer?
For the reaction a(g) 2 b(g), a reaction vessel initially contains only a at a pressure of pa = 1.32...
Questions







Mathematics, 04.11.2020 18:20






Social Studies, 04.11.2020 18:20



Mathematics, 04.11.2020 18:20