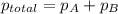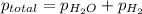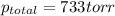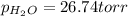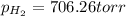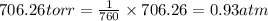Chemistry, 29.07.2019 01:30 dasdsadsafdhgifsdu
Zinc reacts with aqueous sulfuric acid to form hydrogen gas: zn(s) + h2so4(aq) → znso4(aq) + h2(g) in an experiment, 201 ml of wet h2 is collected over water at 27°c and a barometric pressure of 733 torr. the vapor pressure of water at 27°c is 26.74 torr. the partial pressure of hydrogen in this experiment is atm.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:40
Who is better, messi or cristiano, i need this for a chemistry class. asap
Answers: 1

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2
You know the right answer?
Zinc reacts with aqueous sulfuric acid to form hydrogen gas: zn(s) + h2so4(aq) → znso4(aq) + h2(g)...
Questions


Mathematics, 20.09.2020 04:01


Mathematics, 20.09.2020 04:01

English, 20.09.2020 04:01



Health, 20.09.2020 04:01








Mathematics, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01