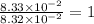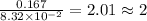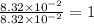Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3
You know the right answer?
What is the empirical formula of a substance that contains 8.33×10−2 mol of carbon, 0.167 mol of hyd...
Questions

Chemistry, 02.03.2020 17:34
















Computers and Technology, 02.03.2020 17:35



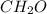
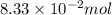
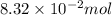
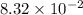 moles.
moles.