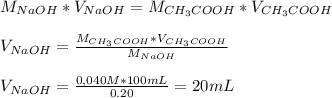Chemistry, 29.07.2019 11:30 natishtaylor1p8dirz
Calculate the volume in ml of 0.20m naoh needed to react completely with 100.ml of 0.040m acetic acid.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2


Chemistry, 23.06.2019 02:00
The plant food contains nh4)3po4 what tests would you run to verify the presence of the nh4 ion and the po4 ion
Answers: 2
You know the right answer?
Calculate the volume in ml of 0.20m naoh needed to react completely with 100.ml of 0.040m acetic aci...
Questions

English, 01.12.2020 18:40

History, 01.12.2020 18:40

Mathematics, 01.12.2020 18:40




Mathematics, 01.12.2020 18:40

Mathematics, 01.12.2020 18:40




History, 01.12.2020 18:40

Mathematics, 01.12.2020 18:40

Physics, 01.12.2020 18:40

Arts, 01.12.2020 18:40

English, 01.12.2020 18:40




World Languages, 01.12.2020 18:40