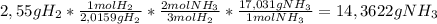Chemistry, 29.07.2019 12:30 lavishbre12
Given the balanced equation 3h2(g)+n2(g)=2nh3(g), calculate the mass of nh3 produced by the complete reaction of 2.55g of h2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
Given the balanced equation 3h2(g)+n2(g)=2nh3(g), calculate the mass of nh3 produced by the complete...
Questions

Biology, 29.08.2019 02:30

History, 29.08.2019 02:30

Mathematics, 29.08.2019 02:30



Arts, 29.08.2019 02:30

History, 29.08.2019 02:30

Mathematics, 29.08.2019 02:30






Physics, 29.08.2019 02:30




Chemistry, 29.08.2019 02:30


Mathematics, 29.08.2019 02:30