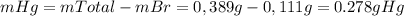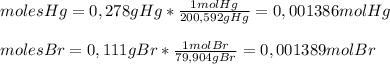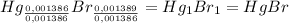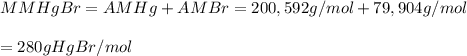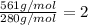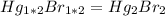Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 23.06.2019 02:20
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2

Chemistry, 23.06.2019 04:20
Which activity describes an application of topographic maps? check all that apply. recreation, such as camping and hiking engineering, such as the construction of roads and buildings science, such as mapping stars in the sky business, such as analyzing population centers science, such as analyzing surface features
Answers: 1
You know the right answer?
Asample of a compound of mercury and bromine with a mass of 0.389 g was found to contain 0.111 g bro...
Questions

Mathematics, 04.06.2021 02:30

Mathematics, 04.06.2021 02:30

Mathematics, 04.06.2021 02:30





Spanish, 04.06.2021 02:30

Mathematics, 04.06.2021 02:30

Mathematics, 04.06.2021 02:30

Spanish, 04.06.2021 02:30


History, 04.06.2021 02:30

English, 04.06.2021 02:30



Physics, 04.06.2021 02:30